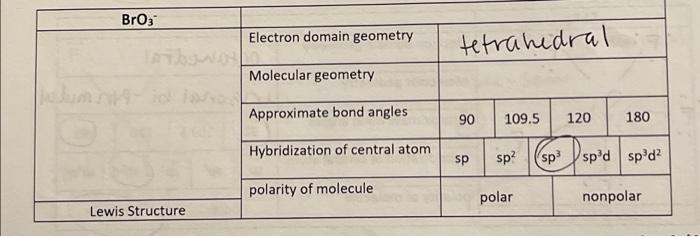
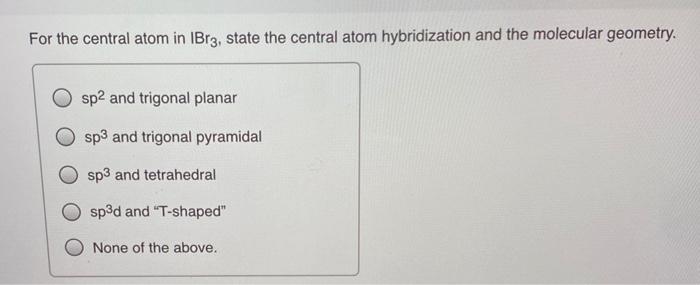
SOLVED: Of the following, the central atom is sp3d2 hybridized only in BeF2, AsCl3, PH3, Br3, O, and XeF4.

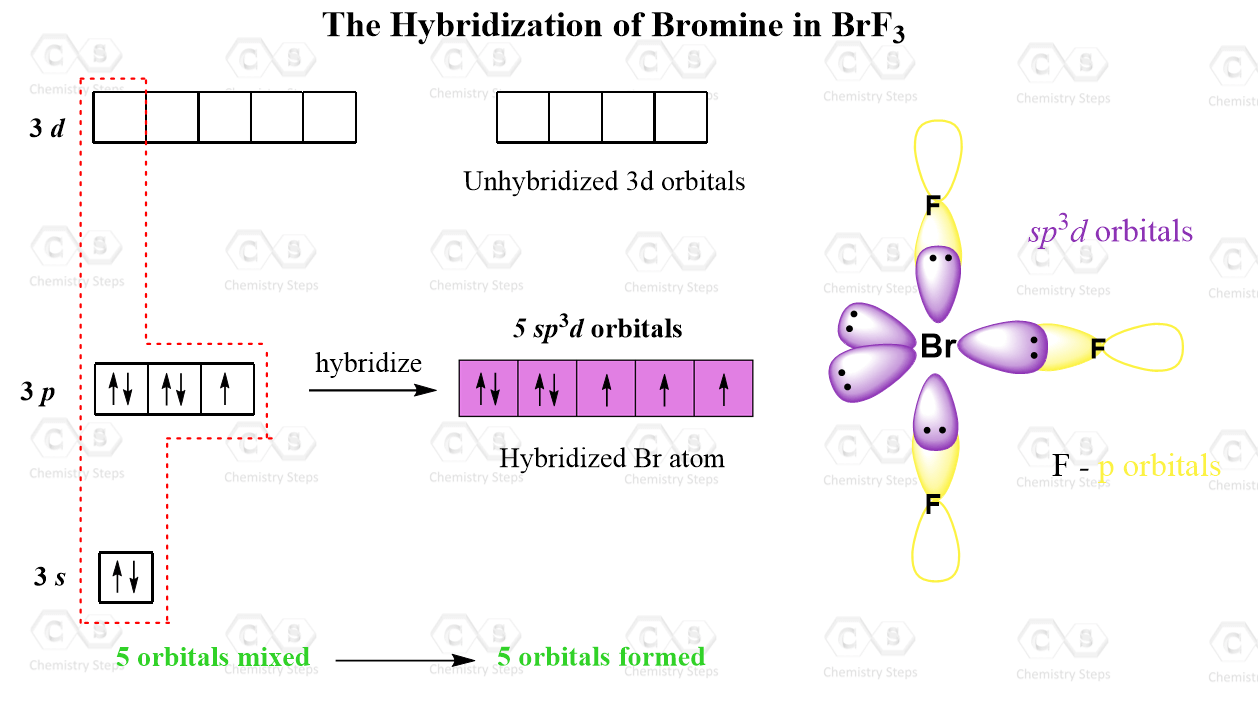
give the structure and mean rion the hybridisation of central atom of the following (a)if7 (b) brf3 (c) - Brainly.in

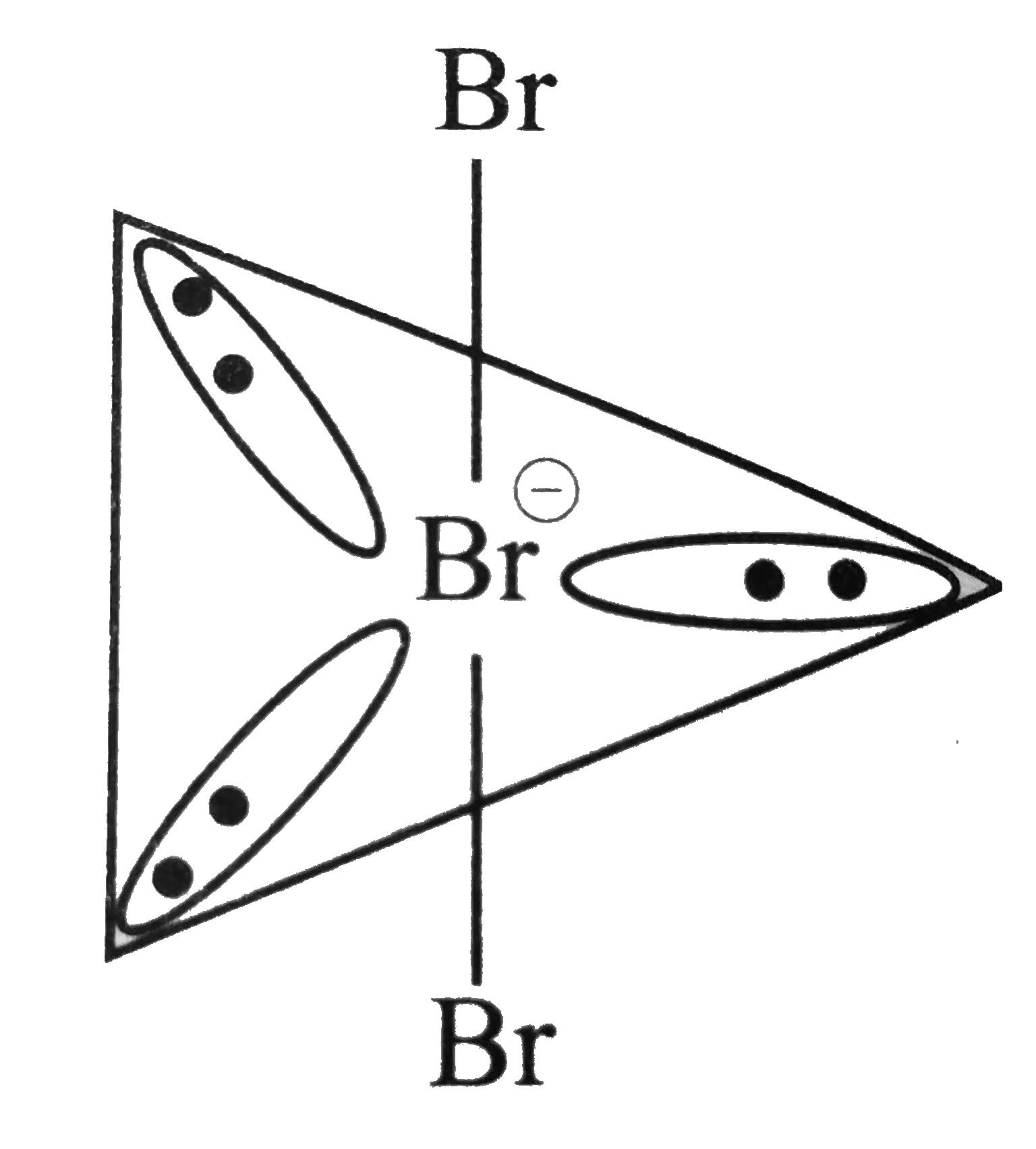
The hybridization and geometry of Br{F}_{3} molecules are:{ sp }^{ 3 }{ d }^{ 2 } and tetragonal{ sp }^{ 3 }d and T-shaped{ sp }^{ 3 }d and bentnone of these
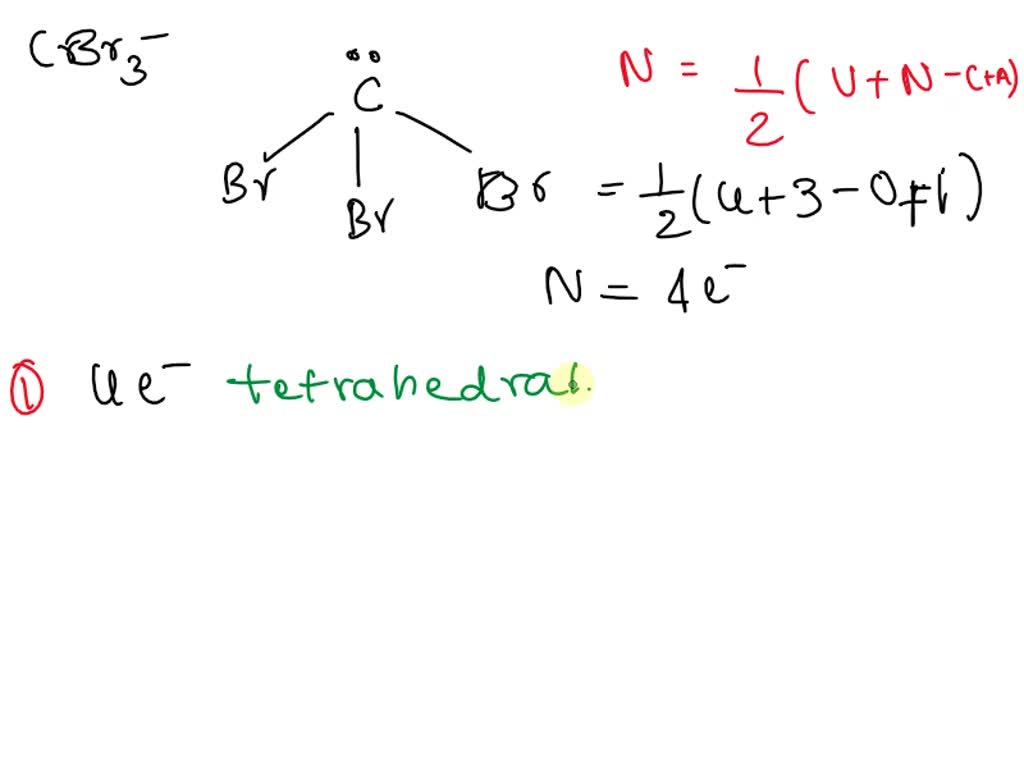
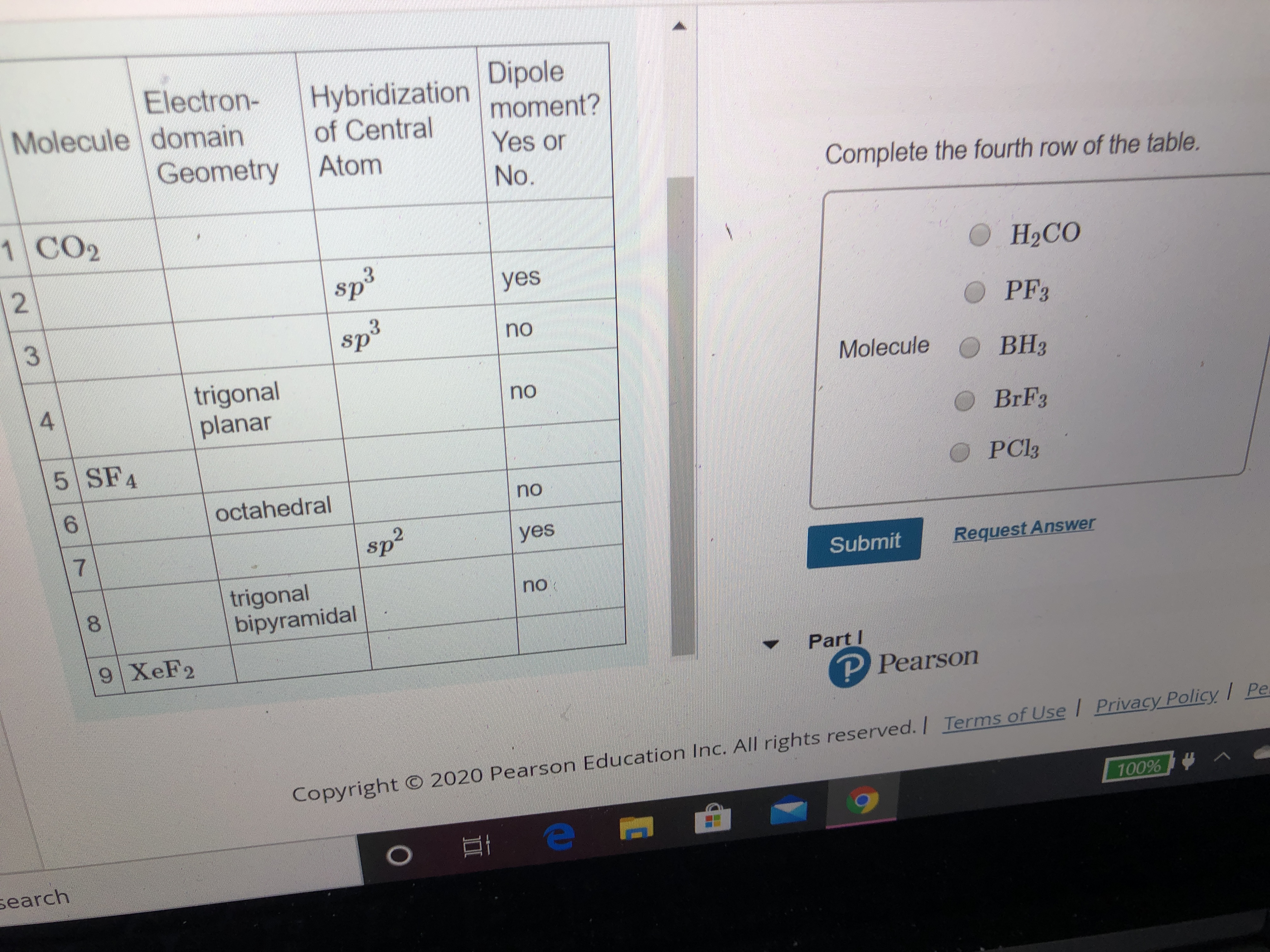
SOLVED: Give the electron geometry, molecular geometry, and hybridization for CBr3-. Electron geometry: trigonal planar Molecular geometry: trigonal pyramidal Hybridization: sp3

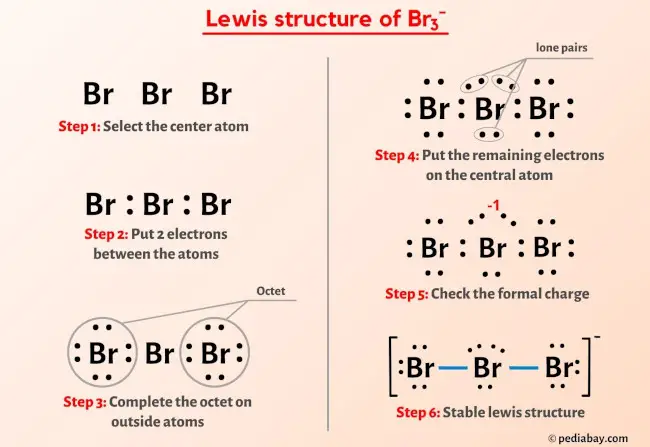
SOLVED: Based on hybridization, what is the polarity of Br3? A) Non-polar B) Ionic C) It cannot be determined. D) Polar Based on hybridization, what is the polarity of Brs? A) Non-polar