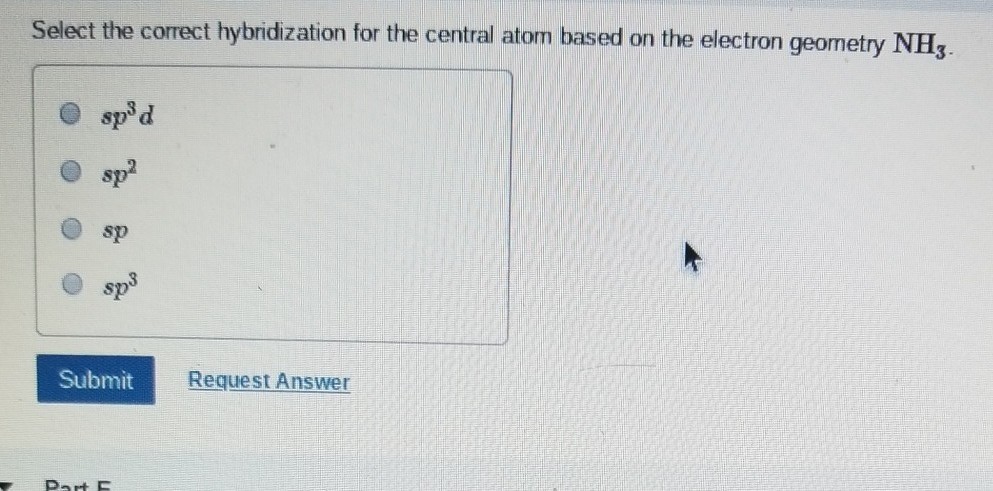
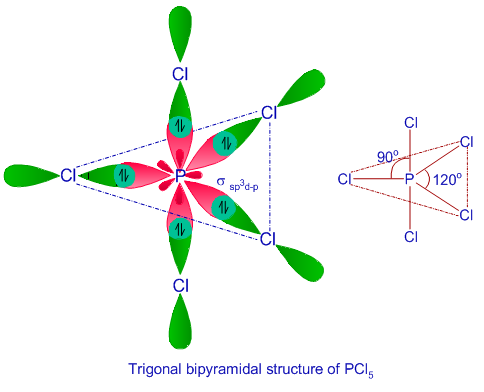
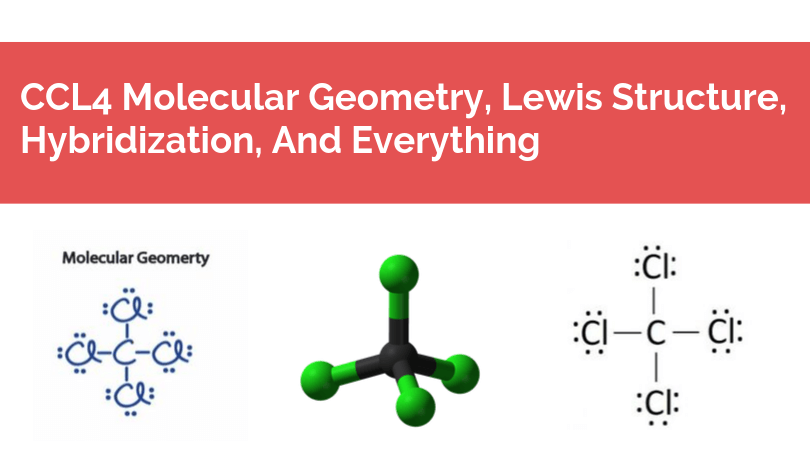
We all know that CCl4 and SiCl4 both compound remains in Tetrahedral structure , with a hybridization of sp³ in the central atom. Now , Si is a much larger atom than
Chemokine-mediated inflammation in the degenerating retina is coordinated by Müller cells, activated microglia, and retinal pigment epithelium | Journal of Neuroinflammation | Full Text

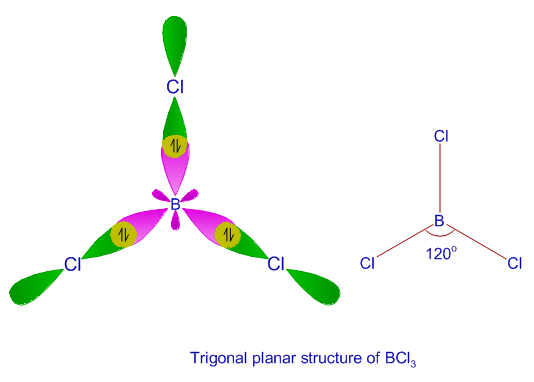
State hybridization in Bcl3 molecule - Chemistry - Chemical Bonding and Molecular Structure - 12421859 | Meritnation.com
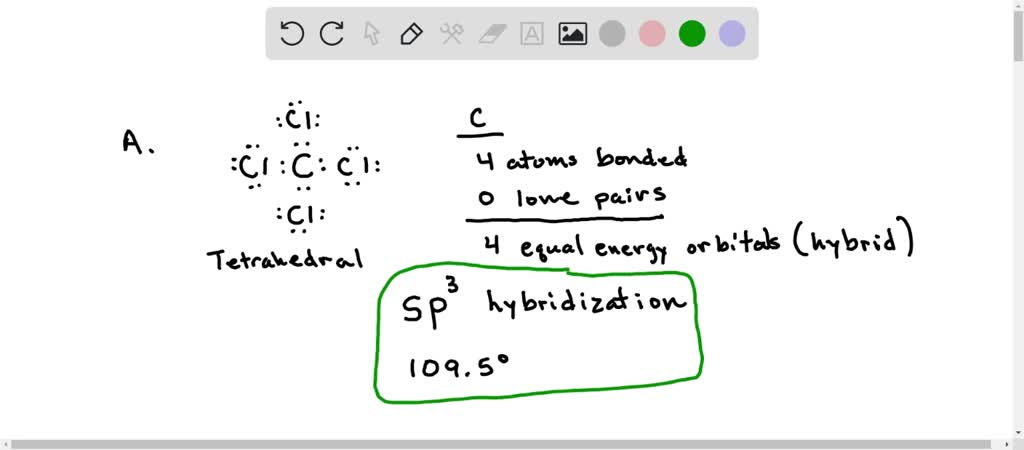
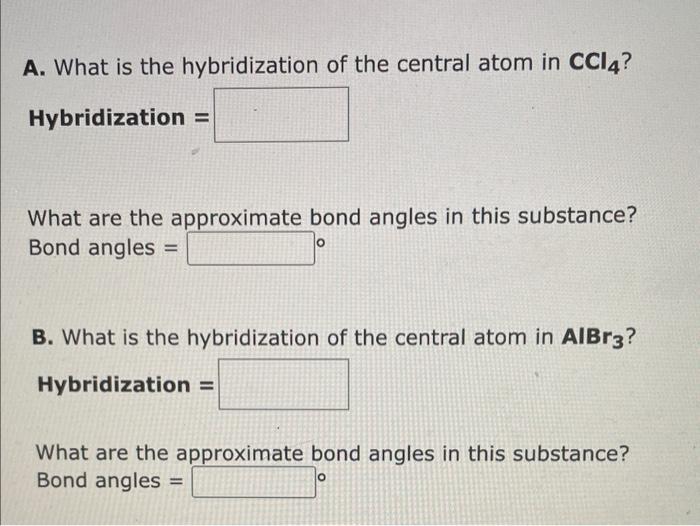
SOLVED: A. What is the hybridization of the central atom in CCl4? Hybridization = What are the approximate bond angles in this substance? Bond angles = ° B. What is the hybridization

SOLVED: The hybridization of the carbon atom in the carbon tetrachloride molecule (CCl4) is of the type sp3.

Starting from Lewis structure, determine the hybridization types of the central atom of TeCl4 and ICl4 .
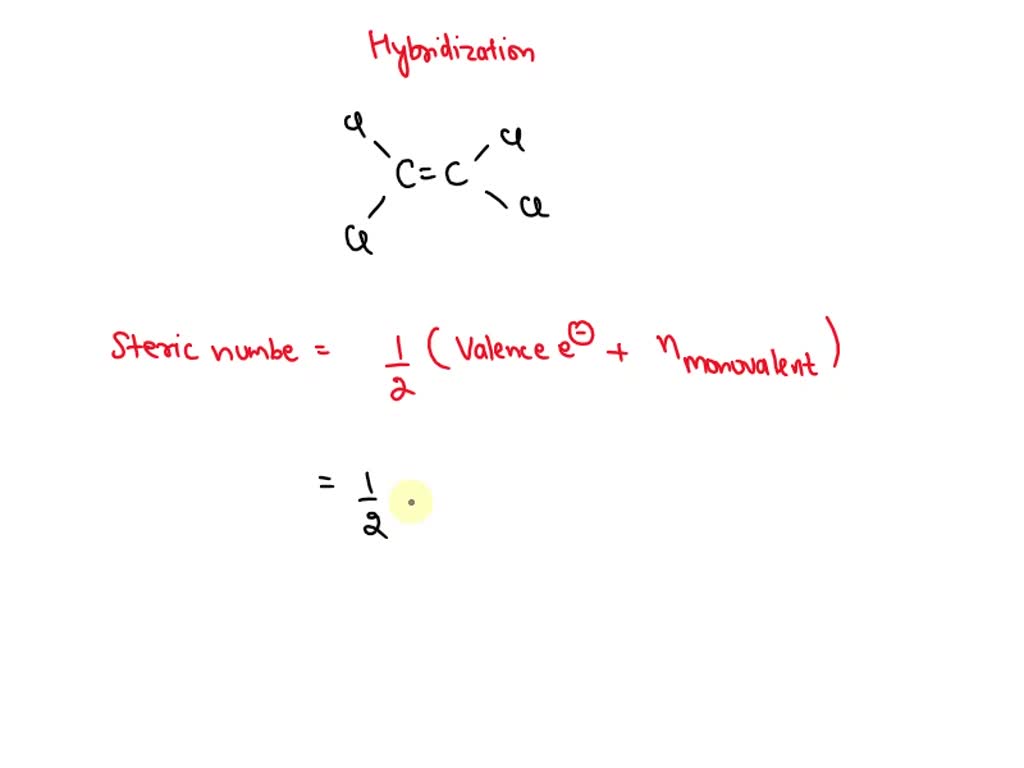
SOLVED: Tetrachloroethylene, a common dry-cleaning solvent, has the formula CCl4. Its structure is C=C-Cl. Use the VSEPR and VB theories to describe the bonding in this molecule. C hybridization: sp3. The double

![Explain hybridization of central atoms in \\[CC{l_4}\\]. Explain hybridization of central atoms in \\[CC{l_4}\\].](https://www.vedantu.com/question-sets/1e78619c-a1a6-4268-a0bc-9c654e3103565131807361122774649.png)