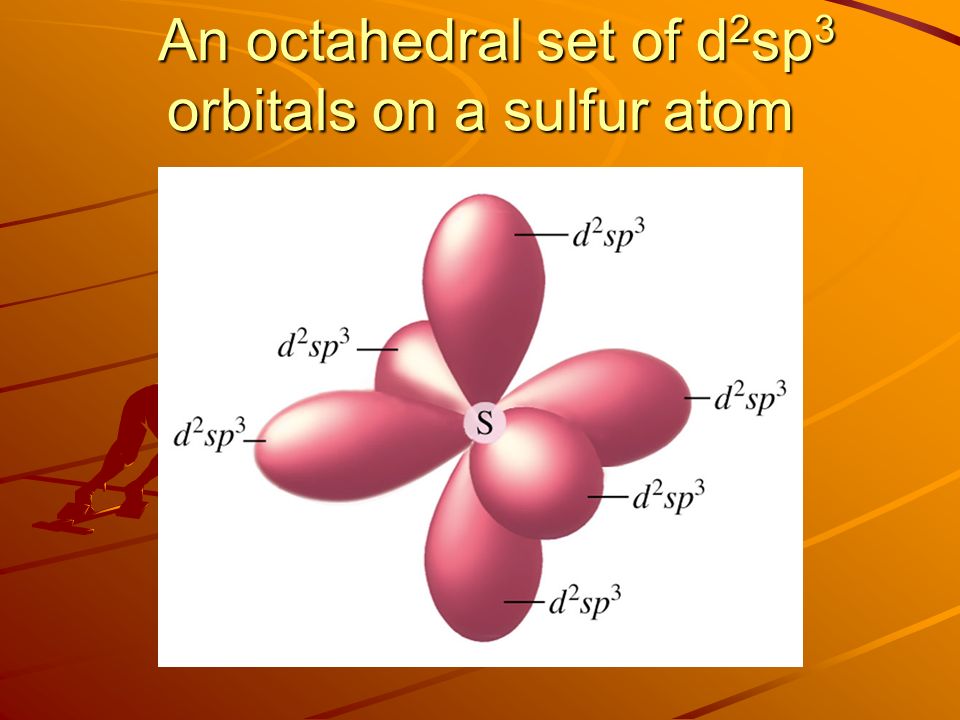
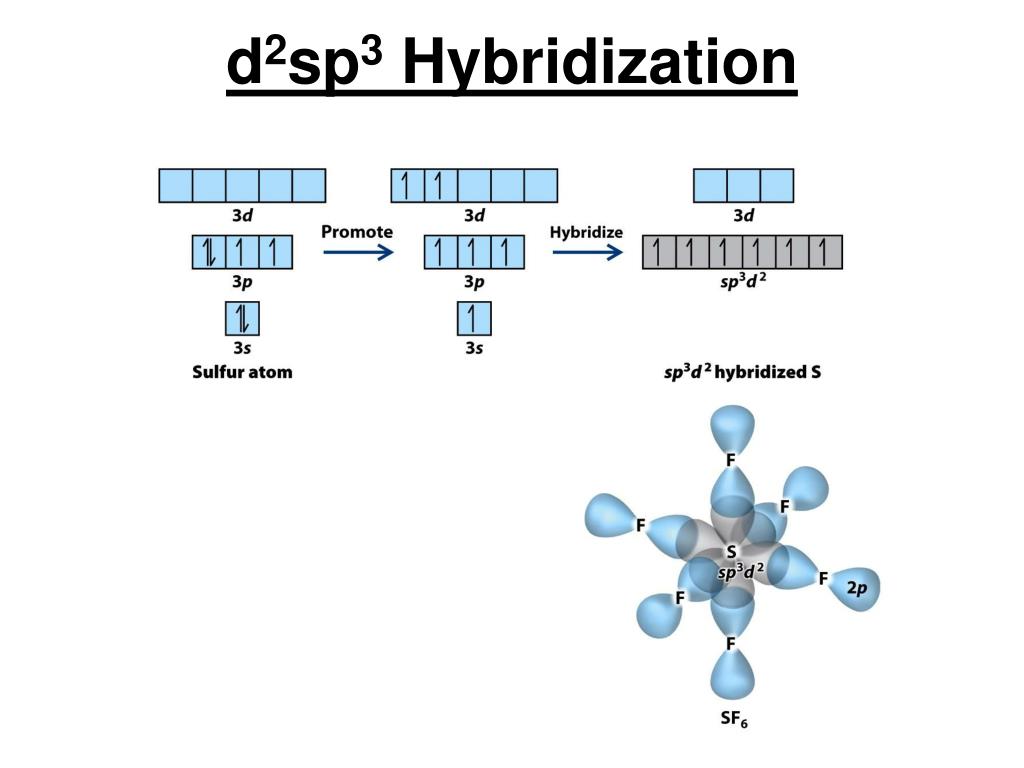
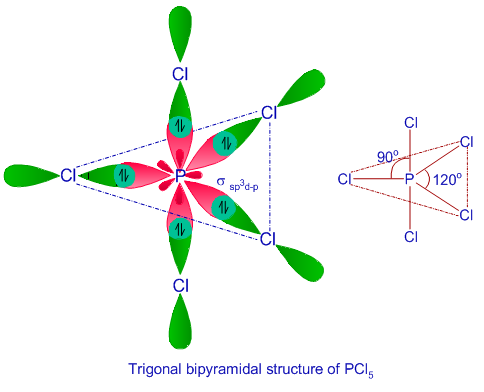
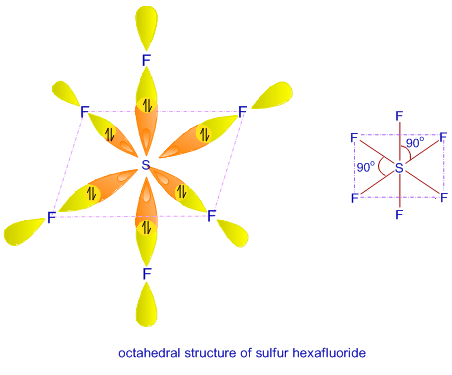
A complex involving d^2sp^{3} hybridization is :a square planar geometrya tetrahedral geometryan octahedral geometrytrigonal planar geometry
Difference Between sp3d2 and d2sp3 Hybridization Key Difference - sp3d2 vs d2sp3 Hybridization What is sp3d2 Hybridization

Which of the following compounds exhibit d2sp3 hybridization? Select "yes" for molecules with d2sp3 hybridization and "no" for all others. KrCl4 TeF6 PBr5 KrCl2 ICl5 IF3 TeCl4 | Homework.Study.com
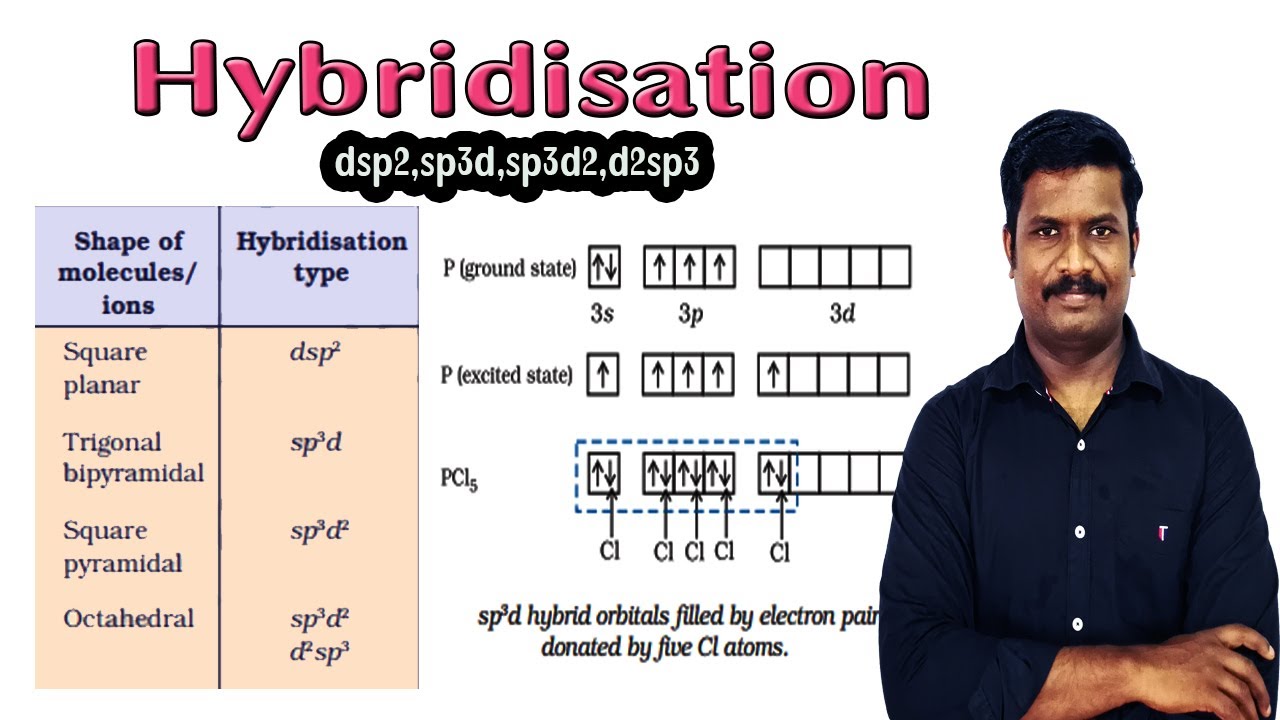
10.Hybridisation | dsp2 | sp3d | sp3d2 | | d2sp3 | Hybridisation Involving d Orbitals | ncert - YouTube



![Hybridisation of [Ni(NH3) 6]2+ is d2sp3 or not?????? - EduRev NEET Question Hybridisation of [Ni(NH3) 6]2+ is d2sp3 or not?????? - EduRev NEET Question](https://edurev.gumlet.io/ApplicationImages/Temp/5610672_cf5cdd6f-a6a7-441d-a688-265da1aa183a_lg.png)