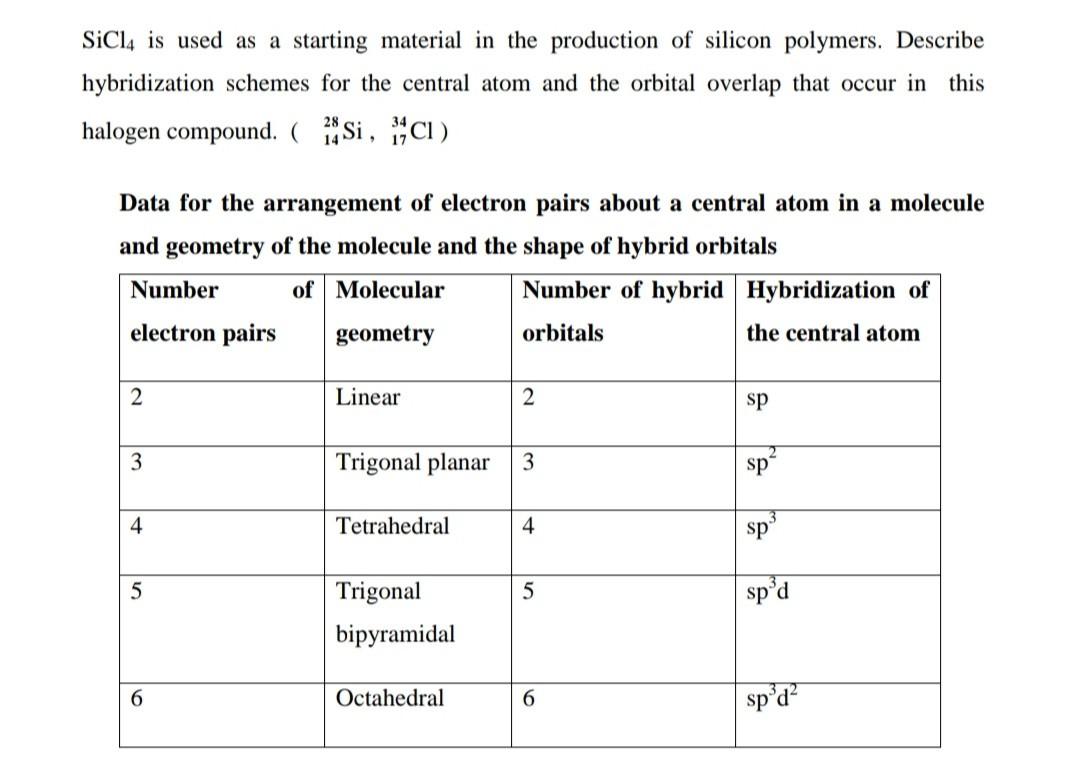
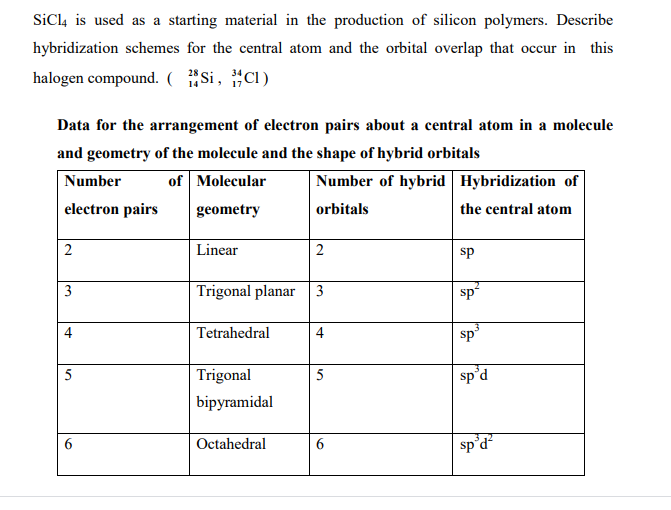
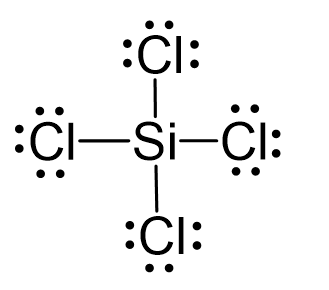
BCU-NEP-IV SEM-DSC-04-Chemical bonding-Valence Bond Theory(VBT)Sp3 Hybridization-SiCl4 as an Example - YouTube
We all know that CCl4 and SiCl4 both compound remains in Tetrahedral structure , with a hybridization of sp³ in the central atom. Now , Si is a much larger atom than
Q. Which of the following pair has same hybridisation. 1] BF3 NF3 2] SF4 SiCl4 3] ClO4 ClO2 4] CO2 SiO2

SOLVED: 4a) Using Valence Bond Theory, show the hybridization and bonding scheme for silicon tetrachloride (SiCl4): (a) write the atomic orbital diagram for the central atom, (b) circle the atomic orbitals that
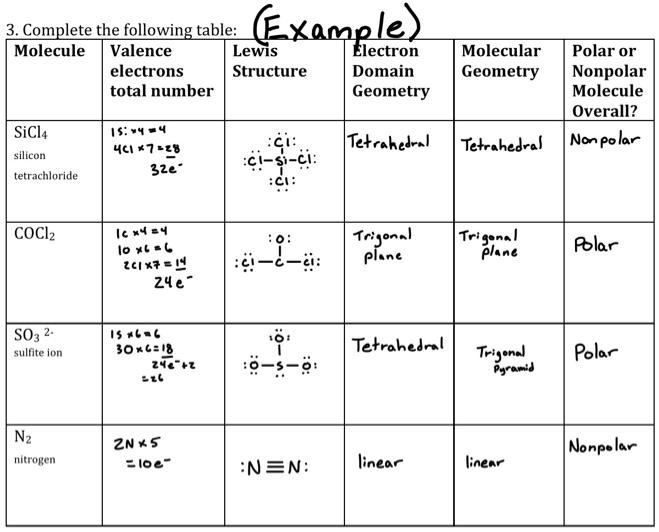
SOLVED: Complete the following table: Molecule Valence Lewis Electrons Structure Domain Total Number Geometry Molecular Geometry Polar or Nonpolar Molecule Overall? Nonpolar SiCl4 Silicon Tetrachloride 4 7-2-4 3-2-4 Tetrahedral Tetrahedral Nonpolar COCl2

SiCl4 lewis structure, molecular geometry, hybridization, polar or nonpolar | Molecular geometry, Molecular shapes, Molecular
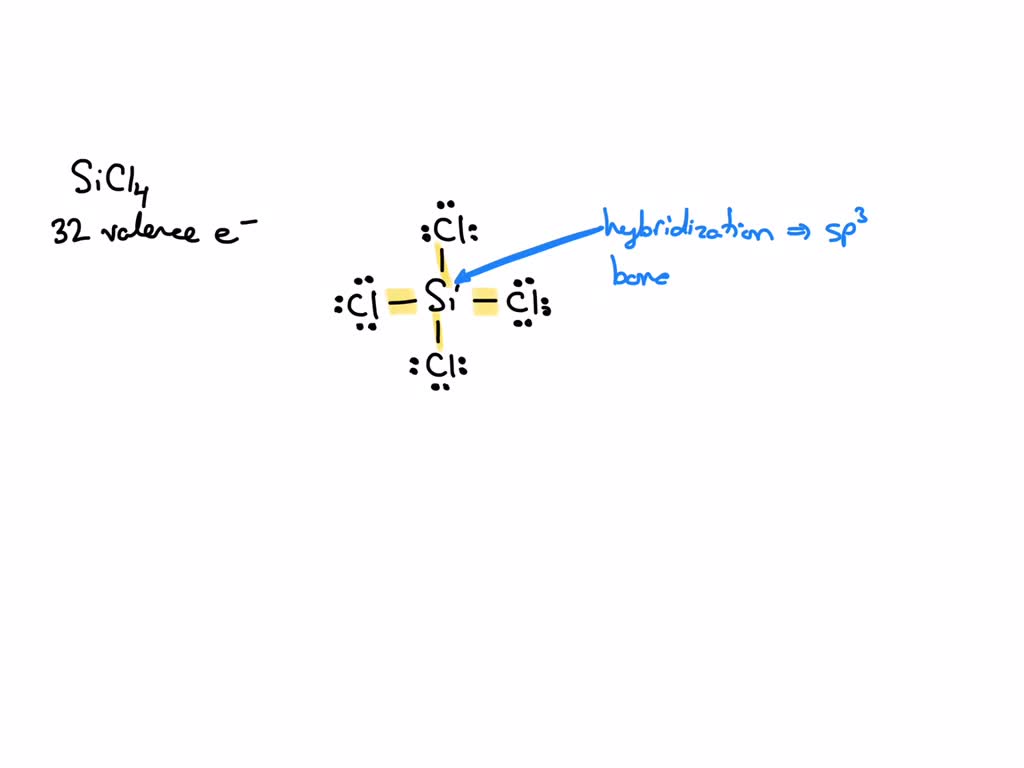
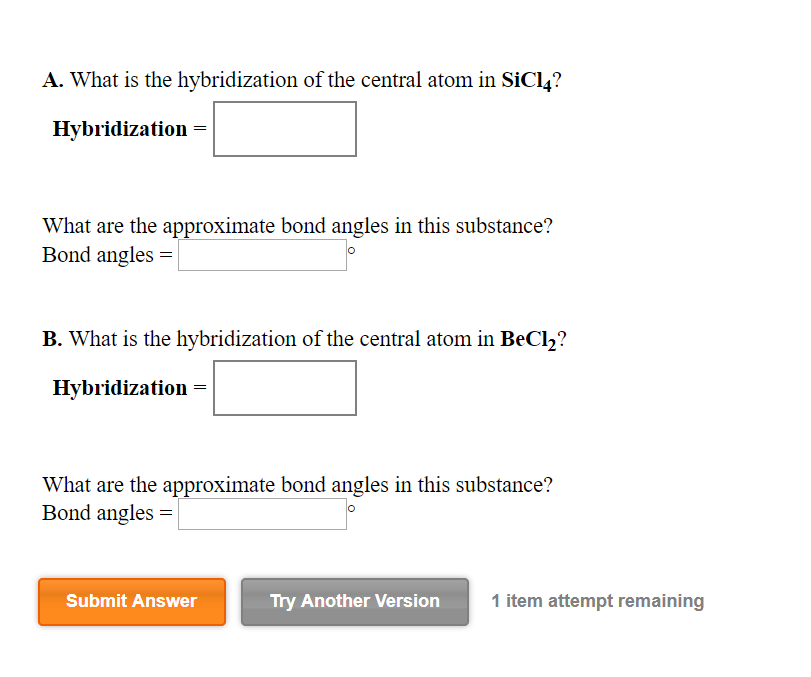
SOLVED: What is the hybridization of the central atom in SiCl4? Hybridization = What are the approximate bond angles in this substance? Bond angles = fill in the blank 2° B. What
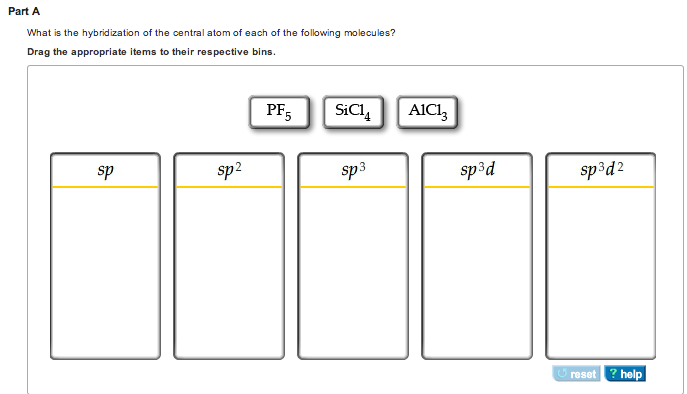
What is the hybridization of the central atom of each of the following molecules? - Home Work Help - Learn CBSE Forum
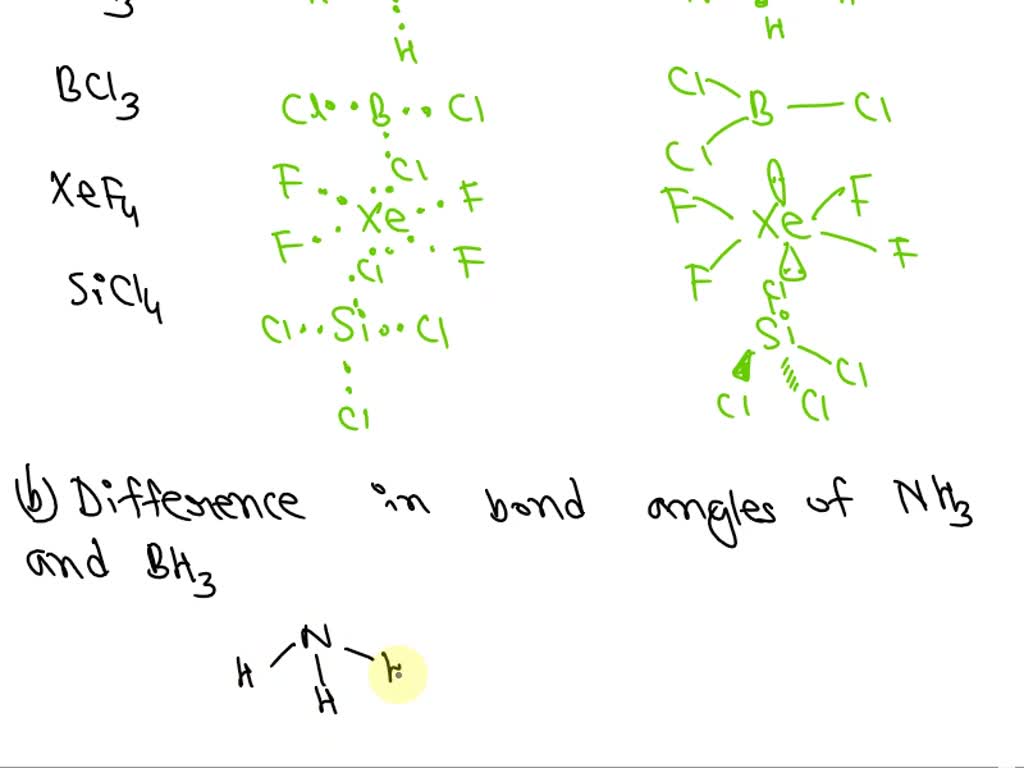
SOLVED: a) Draw the Lewis structures and molecular shapes of NH3, BH3, PCl5, XeF4 and SiCl4, and indicate the hybridization types and geometric shapes. b) Compare the bond angles in NH3 and
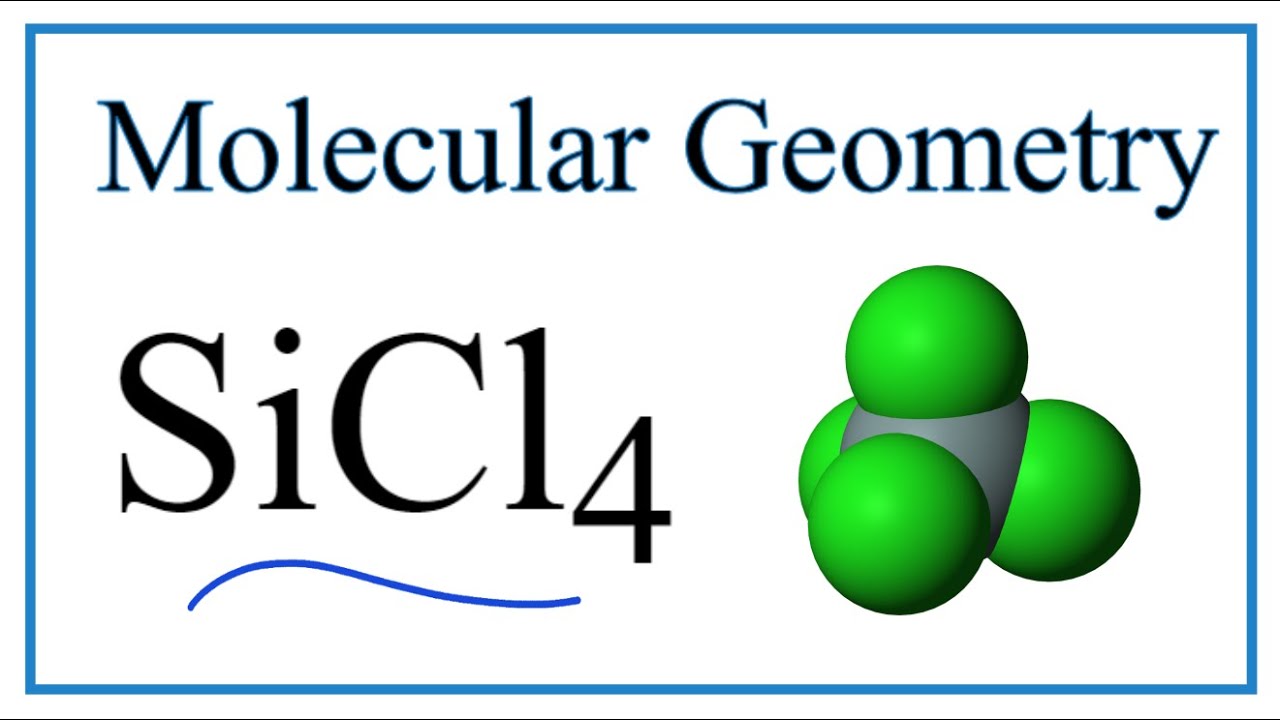
Silicon tetrachloride SiCl4: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure –
sp^3 (sp three) Hybridization (Tetrahedral hybridisation). - Sarthaks eConnect | Largest Online Education Community